Cell-ID International MD-PhD/PhD Call
The applications for the 2026 call are now closed

MD-PhD/PhD

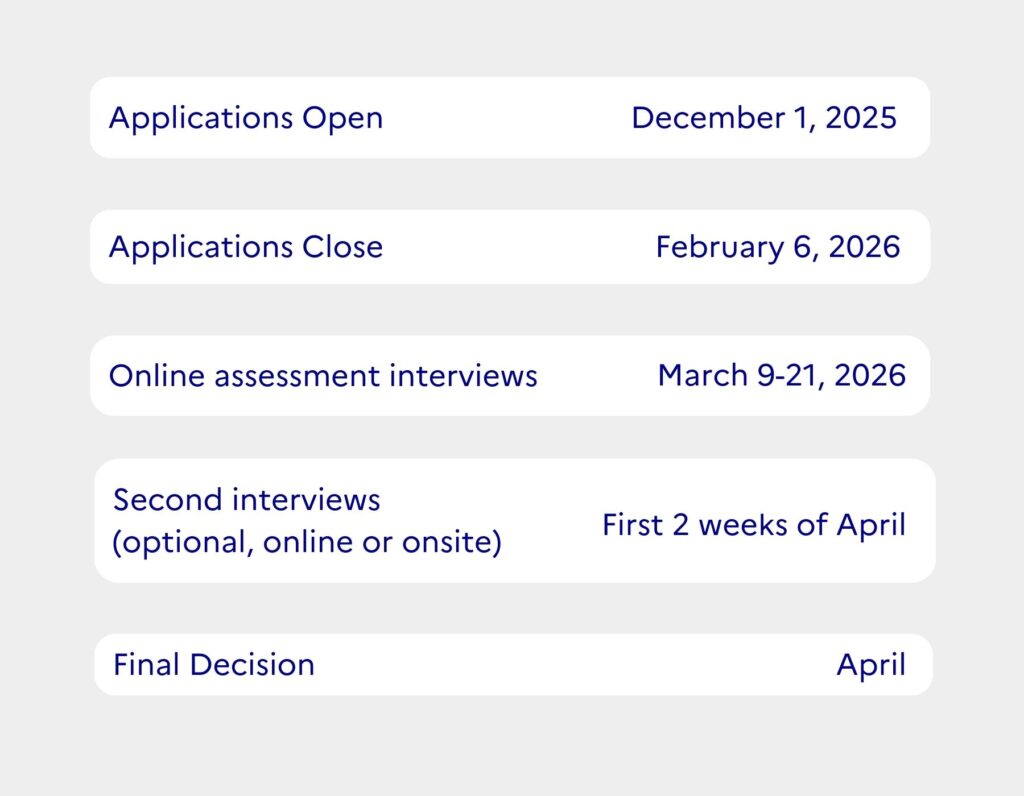
Deadline February 6 2026, 6PM (CET)

France
Join the Cell-ID (Cell Identities and Destinies) Research Program – MD-PhD/PhD Positions Starting 2026
Are you an outstanding, creative, and motivated young researcher? Join Cell-ID, a multidisciplinary program uniting 29 French laboratories in a collaborative effort to understand how cells shape normal development, how these processes are disrupted in pediatric brain cancers, and how this knowledge can pave the way toward a cell-based interceptive medicine.
About Cell-ID
The Cell-ID program focuses on uncovering the molecular mechanisms that govern cell fate determination during neural development and how their disruption contributes to childhood brain cancers.
Our goal is to design strategies for a cell-based interceptive medicine: targeting the earliest cellular and molecular events in cancer to prevent disease progression.
Led by CNRS and Inserm, in collaboration with major French institutions and international partners, Cell-ID integrates omics technologies, advanced imaging, and computational modeling to study genome function and 3D nuclear organization in neural tissues under normal and pathological conditions. By combining cutting-edge experimental tools with predictive models, the program aims to define and modulate cell trajectories during neural development.
Timeline

Cell-ID’s PhD Training proposal
- Interdisciplinary Core Training: Deep learning, statistics, data analysis, developmental biology, epigenetics, cancer biology, and state-of-the-art technologies (RNA-seq, advanced microscopy, etc.).
- Soft Skills Development: scientific writing, bioethics, unconscious bias, sustainability in research, and diversity, equity, and inclusion (DEI).
- Holistic Approach: Preparing students to become independent scientists and engaged citizens.
- Program Structure: Three years to complete the thesis, with optional curricular activities. A 4th year may be considered upon request and supervisor approval.
What We Offer
- Dual Supervision: Each student is co-supervised by two PIs from different disciplines, ensuring a cross-disciplinary perspective.
- Comprehensive Training: Technical courses plus transferable skills workshops.
- Lab Rotations & Collaborations: Opportunities to work across multiple labs and approaches.
- Unique Environment: Leading labs and institutions with access to advanced technical facilities.
- Community Engagement: Annual PhD retreats, conferences, bootcamps, and interdisciplinary projects.
- Funding options:
- Standard 3-year PhD fellowships (for non-medical or medical students in their 4th year)
- Two-year funding for MDs, enabling 2 years of full-time lab work followed by a third PhD year conducted alongside hospital duties
Paired Labs with Available MD-PhD/PhD Positions

Computational Systems Biology of Polycomb regulation in pediatric brain cancers
Giacomo Cavalli and Daniel Jost
The Polycomb machinery is essential in regulating gene silencing during embryogenesis and development, through the coordinated action of transcription factors, histone modifying enzymes and architectural proteins. The initiation of several pediatric brain cancers has been associated with perturbations in such a machinery, which have important system-scale consequences on gene regulation. While the molecules whose functions may be impaired have been identified, how such an impairment affects mechanistically gene expression locally and at the systems scale remains elusive.
Read more
Using mathematical modeling and data analysis in close collaboration with experimentalists, we aim at characterizing Polycomb regulation in normal and pathological conditions in order to better understand the systemic relation between metabolism, Polycomb machinery, genome organization and gene expression in the context of neural development. This project is a direct collaboration between the groups of Daniel Jost (CNRS, ENS de Lyon; modeling) and Giacomo Cavalli (CNRS, IGH, Montpellier; experiments). The student will be mainly located in Lyon but will visit regularly the Cavalli’s group to foster exchanges with experimentalists. More broadly, this project is part of a national effort to characterize cell derailment in pediatric brain cancer via the consortium Cell-ID.
Identifying the determinants of SMARCB1-mediated cell transformation
Franck Bourdeault and Raphaël Margueron
The leading genetic cause for Atypical Teratoid/Rhabdoid Tumors is the mutation of Smarcb1. Yet, inactivation of this gene in many cell lines impairs cell proliferation and is strongly counter selected (Depmap essential in 971/1186 cell lines). Moreover, we have shown that a specific developmental context is required for SMARCB1 to promote cell proliferation. This raises the question of what control the cellular outcome of SMARCB1 inactivation.
Read more
To tackle this question, we propose to engineer various models of acute inactivation of Smarcb1 (CRISPRi & degron) and to perform genetic screens to identify genes whose altered expression enable cells to survive the deletion of SMARCB1. We will then investigate how those genes are regulated during development, in particular at the phase when ATRT is thought to arise. We will manipulate their expression in ATRT cell lines and PDX to determine their contribution to tumorigenesis. Finally, we will use our model of SMARCB1 acute degradation to investigate its direct and early targets genes (CUT&RUN, TT-seq, HiChIP, Proteome) and further investigate its mechanism of action.


Investigate the metabolomic dependencies in medulloblastoma
Alexandre Baffet and Olivier Ayrault
Deregulations in biological networks are key events in pathogenesis of cancer, whose knowledge should open the road to novel therapeutic strategies. In medulloblastoma (MB), the most common malignant pediatric brain tumor arising from the cerebellum, these network deregulations are not fully understood, and therapeutic success is still limited. MB is a heterogeneous disease; current genetically-defined subgroups include Wingless (WNT)-activated, Sonic Hedgehog (SHH)-activated and non-WNT/non-SHH. This last category corresponds to the established molecular subgroups “group 3” and “group 4” and accounts for the majority of MB cases. Studies have also provided genomic, epigenetic and transcriptomic landscape of human MB samples, nevertheless they have not yet attained the global comprehension of MB complex dynamic networks.
Read more
Building on previous studies (Forget et al., Cancer Cell, 2018; Hovestadt et al., Nature Reviews Cancer, 2020), we recently have performed a proteomic-metabolomic analysis (including genome (WGS), epigenome (850K), transcriptome (RNA-seq), proteome (mass spectrometry (MS)), phosphoproteome (MS), metabolome (MS-based)) and associated clinical data) on our cohort of 384 MB patient samples. Interestingly, through extensive integrated proteogenomic and metabolomic analyses across all MB, we unveiled a distinct metabolic signature in the most aggressive subgroup of MB, at both proteome and metabolome levels.
Given these results, the proposed project aims to further investigate this metabolic dependency. More specifically, we will:
Aim 1: Characterize this signature across both normal development and MB contexts
Aim 2: Decipher the cellular and molecular mechanisms underlying this signature in MB
This work will involve the use of several cutting-edge approaches such as single cell sequencing, proteomics or advanced microscopy.
Overall, this project should contribute to a greater characterization of metabolic vulnerabilities in MB and thus, may pave the way to the development of promising therapeutic avenues in the worst outcome MB subgroup.
Long-Read Single-Cell and Spatial Transcriptomics of the Developing Human Hindbrain
Pascal Barbry and Alain Chédotal
This proposal aims to characterize human hindbrain (Cerebellum and brainstem) development using an integrated pipeline combining long-read nanopore sequencing (ScNaUmi-seq and SiT) with spatial transcriptomics, to better understand the cellular origins of medulloblastoma. We will shift from gene-centric to transcript-centric paradigms by capturing full-length sequence heterogeneity and splicing diversity lost in standard workflows. Our experimental approach combines single-nuclei/single-cell RNA sequencing with dual spatial methods: SiT for global isoform mapping and Xenium for precise cell-cell interaction analysis on adjacent tissue sections. Targeted in situ sequencing will generate spatial maps enabling probabilistic cell mapping and 3D reconstruction from optically cleared specimens or tissue section processed with multiplex immunostaining. We will develop AI-driven computational frameworks for multi-omics integration and annotation, incorporating Human Cell Atlas resources.
Read more
Methods will be optimized for rare embryonic samples to capture long transcripts. Using unique access to human embryonic tissue, we will map neuronal progenitor lineages and their spatial organization during brainstem development. By establishing a comprehensive developmental atlas, we aim to identify the progenitor populations susceptible to malignant transformation in medulloblastoma, the most common pediatric brain cancer with no cure to date.
This project is a direct collaboration between the team of Pascal Barbry (Nice) and Alain Chédotal (Institut de la Vision Paris et MeLIS, Lyon) who will co-supervise the student. The student will be mainly located in Paris but will visit regularly the Barbry lab. This project is part of a national effort to characterize cell derailment in pediatric brain cancer via the consortium Cell-ID.


4D investigation of cellular interaction in a Drosophila model of ATRT brain tumours
Pauline Spéder and Christophe Zimmer
Atypical Teratoid Rhabdoid Tumours (ATRT) are highly aggressive pediatric brain tumours primarily driven by the loss of the chromatin remodeling factor SMARCB1. However, the role of the surrounding microenvironment in tumour progression remains poorly understood. To address this, we take advantage of a Drosophila model in which inactivation of snr1 —the fly homolog of SMARCB1— during development induces ATRT-like tumours. This model offers distinct advantages, including well-defined cells of origin, characterized developmental programs, and advanced genetic engineering.
Our research will focus on deciphering the interactions between tumour cells and the host tissue, including neurons, glial cells, and the blood-brain barrier. We will examine host cell responses at multiple levels —structural organisation, cellular processes, gene expression, epigenetic modifications and metabolic changes— to uncover how these factors influence tumour progression.
Read more
To achieve this, we require high-resolution, multi-colour imaging techniques to map intricate 3D spatial relationships between host and tumour cells throughout tumour progression. To overcome current technical limitations, our two laboratories are collaborating to develop, refine, and validate a novel imaging system combining hyperspectral imaging with light-sheet illumination. This approach will enable rapid, high-resolution imaging of thick tissue samples with minimal preparation, supporting the detection of four or more fluorescent markers simultaneously.
This cutting-edge imaging platform will allow us to precisely map the dynamics of cellular interactions between host and tumour cells, with the aim to improve our understanding of ATRT biology.
Imaging transcriptome evolution in live cells
Edouard Bertrand and Florian Muller
Single cell sequencing has revealed that cells differentiate along well-defined trajectories in the transcriptomic space. However, how the transcriptome evolves in real-time is poorly understood. The goal of the PhD will be to develop imaging-based methods to better apprehend the dynamic regulation of the transcriptome. To this end, transcription will be imaged using a combination of live and fixed cell methods during the differentiation of stem cells. Transcription will be visualized at both the global and gene-specific levels and the data will then be used to reconstruct the trajectories of single cells in real time.

Application Process
Eligibility Criteria
- We welcome applications from excellent and highly motivated students of all nationalities
- Applicants must hold (or expect to obtain before the start of program) both a BSc and MSc (or equivalent, usually 5 years of study) in science, medicine, or related fields.
- There is no application fee.
How to Apply
- Online Form
- Complete in English by or before February 6th, 2026, 6PM (CET)
- Lab Selection
- Choose up to 3 paired labs of interest in order of priority.
- Research Experience
- Describe up to 2 research projects (past or ongoing, that lasted at least 4 weeks each), including aims, methods, and results.
- References
- Provide 2–3 professional references. They should be independent researchers (e.g., supervisors, group leaders); classmates and postdocs should not be listed as referees. References will be contacted after submission and must submit evaluations by February 11th, 2026. Please ensure that your referees are aware of this deadline and are prepared to submit their references on time.
Note: The earlier you submit your application, the sooner your referees will be contacted
- Provide 2–3 professional references. They should be independent researchers (e.g., supervisors, group leaders); classmates and postdocs should not be listed as referees. References will be contacted after submission and must submit evaluations by February 11th, 2026. Please ensure that your referees are aware of this deadline and are prepared to submit their references on time.
- Required documents to upload in the form
- CV (up to 10MB)
- Motivation letter (1 page, up to 10MB)
- Degree certificates (For each degree obtained after highschool, provide the official certificate and the grades. If not in English or French, include a translation. Compile all certificates in a single pdf file of up to 20Mb)
- Submission
- Submit through the online form only—no other methods will be accepted.
Selection process
- Step 1 – Written Application Review: Completeness and eligibility, then scientific evaluation by Cell-ID faculty. Results within ~3 weeks.
- Step 2 – Online Panel Interview: eligible candidates will participate in a 40-minute interview including a 10-minute project presentation and article discussion. Candidates will be assessed by defined criteria that will be communicated to the candidates prior to the interview.
- Step 3 – Optional 1:1 Interview: Top candidates may be invited for additional discussions (online or onsite).
Commitment to Diversity
The Cell-ID PhD program is deeply committed to equity, inclusion, and diversity. We believe a diverse community is not only a moral imperative but also the strongest driver of creativity and scientific excellence.
Funding and contracting arrangements
- Doctoral candidate remuneration according to current funding scales.
- Operating expenses directly related to the research project (consumables, access to databases, software, specific computer equipment, travel for conferences and research missions).
- Contribution to the costs of the doctoral candidate’s additional training.
General provisions
- The doctoral candidates selected under this call for projects will be recruited by a French higher education or research institution (university, doctoral school, Inserm, CNRS, CEA, INRIA, INRAE, etc.), which will act as the managing body for the funding allocated by the PEPR Cell-ID.
- The doctoral candidate will be enrolled in a French doctoral school, which will oversee the administrative and academic supervision of the PhD.
- The doctoral contract will be established for a duration of three years, in accordance with current legal regulations in France. The doctoral candidate will hold employee status and will receive remuneration according to current funding scales.
Commitments of the awardees
- Publications and dissemination
Research work resulting from funded PhD theses must be published in open access, in accordance with the open science policies of the PEPR Cell-ID. - Participation in PEPR Cell-ID activities
Doctoral candidates must participate in seminars, training sessions, and events organized as part of the program. - Project monitoring
Awardees and their managing institution must provide an annual report presenting the progress of the research work, the results obtained, and the use of the allocated funding.
For any questions, please contact phd.applications.cell-id@curie.fr / contact@pepr-cell-id.fr
In application of the GDPR and the Loi Informatiques et Libertés, all candidates have the right to access, rectify, oppose and delete their personal data, to exercise this right, please write to us at the following email address: contact@pepr-cell-id.fr